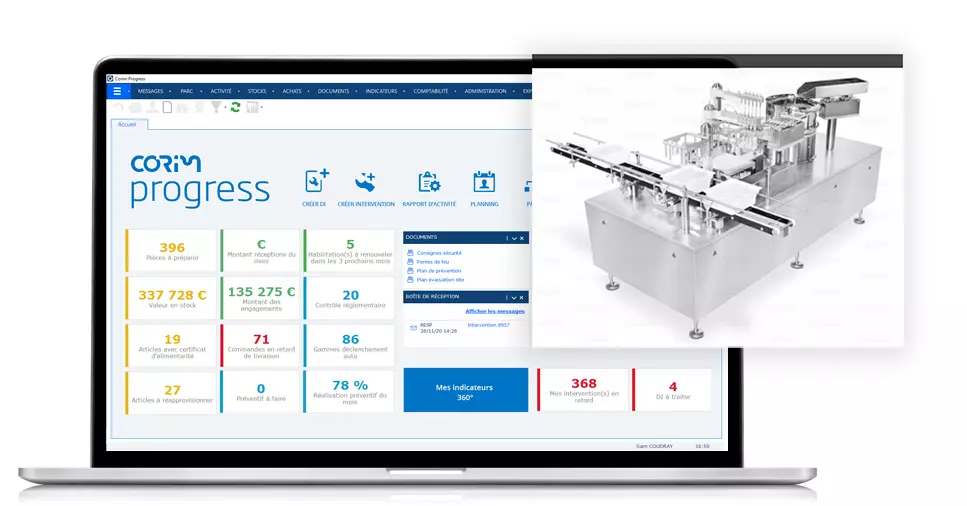
CMMS CORIM PROGRESS
Easily trace and record maintenance information
Prepare your maintenance works taking into account safety aspects and respecting the prevention plan. Manage your expenses and measure your activity thanks to simple and customizable reports. Save time to concentrate on your business.
| SECURITY | LIFE CYCLE COST | AUDIT APPROVED |